Non Conformance Management Software for Manufacturing Business
Identify, Track, and Manage Rejected Parts and Materials With Ease
How rejected, failed, or defective non conformance material is identified and separated within the Manufacturing process is critical to prevent this material from being further processed.
A quick and efficient effort to disposition non conforming material is critical to the quality and consistency of the process, product, or service.
Key Principles of Non Conformance Process
- Identify Non Conformance Product - Example: Tag, Labels, Package, Sort
- Seperate, Segregate - Physically sort, seperate, segregate from Good Product
- Disposition of Non Conformance - Rework, Scrap, Use As-Is, Return To Vendor
- Complete Disposition Action(s)
- Verify Disposition Action(s) are Completed Accurately and Thoroughly.
MUST BE A FULL CIRCLE PROCESS!
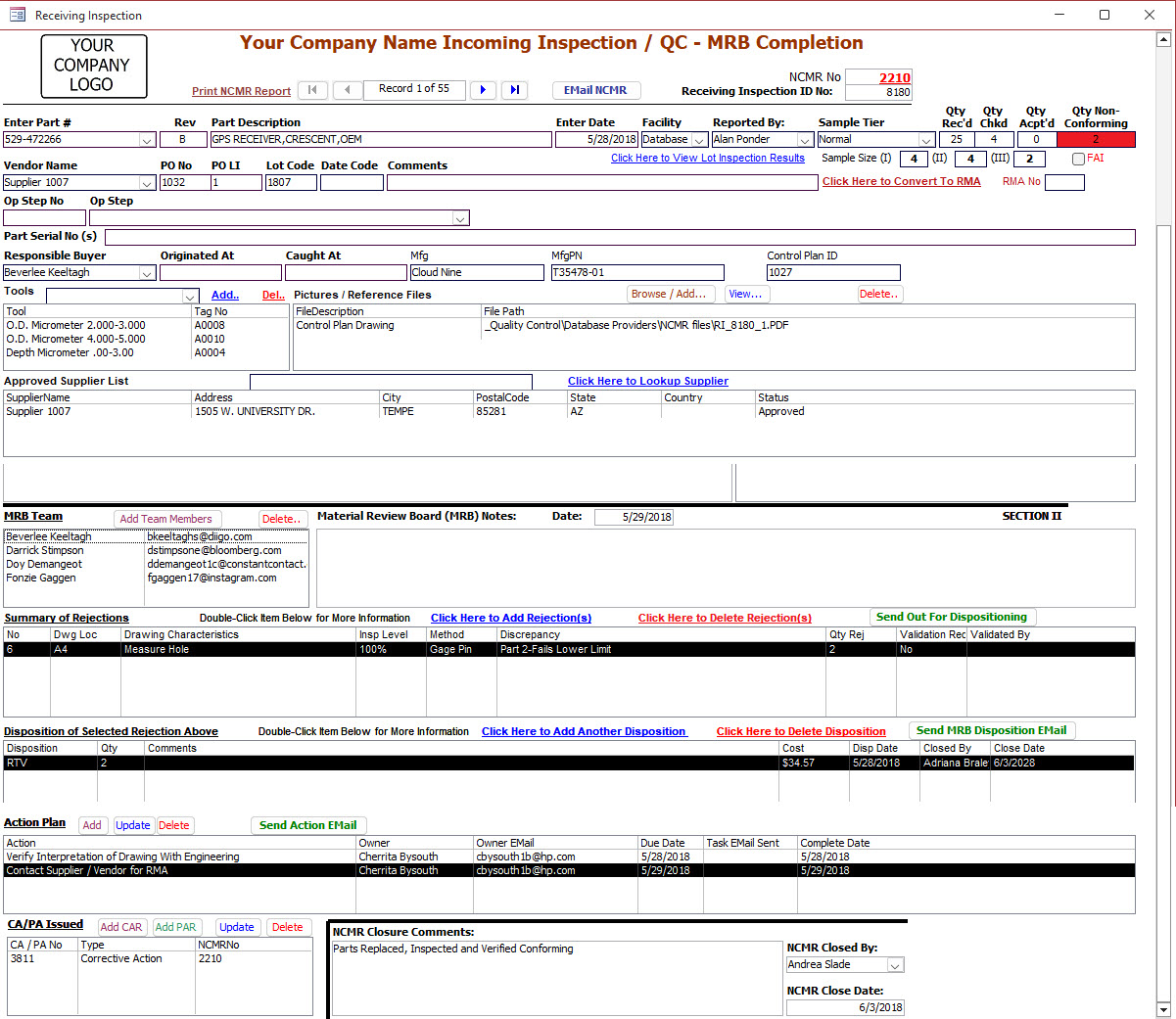
Our Non Conformance Management Software Features
Full Circle Process
- Identify Non Conformance - Part Number, Revision Level, Quantity, Discrepancy
- Segregate, Separate - Label, Tag, Sort, Package, Shelve,
- Send Out For Dispositioning
- Record Disposition Action(s)
- Execute Disposition
- Verify Disposition Actions Complete
- Close Non Conformance Record
Link Documents / Files to Non Conformance Record
- Drawings
- Emails
- Pictures of Defects
- Purchase Order Requirements
- Testing Results
Tool Identification
- Add a List of Calibrated Measuring Devices used for Inspection
- Alerts if the Calibrated Measuring Device has Expired Calibration
Approved Supplier Details
- Automatically Appends Current Supplier's Approval Status
- Ability to Add Additional Suppliers such as Outside Processing or Testing
Electronic Dispositioning Review Process
- Single Click to Generate Email to Dispositioning Parties
- Review and Approval Passwords for Disposition Acceptance
- Single Click To Generate Non Conformance Closure
Continuous Progress to Closure
- Automated Emails to Encourage Progress Through Non Conformance Closure
- User To-Do List Reminders
- Unavoidable Alerts of Open Non Conformance's
Multiple Defect Identifications
- Define all Defects of Non Conformance Lot
- Multiple Disposition Actions Per Lot - Some Rework, Return-To-Vendor, Scrap
Defect Type Reporting
- Define and Group Defects by Type and Process
- Receiving Inspection
- In Process (WIP) Inspection
- Final Inspection
- Customer Returns (RMAs)
Corrective Action Initiation
- Single Click to Initiate and Populate New Supplier or Internal Corrective Action
Robust Reporting / Metrics
- Open Non Conformances
- Key Process Indicators (KPIs)
- Quality Metrics
- Cost of Poor Quality
- Management Review Metrics
- Trends / Top X Non Conformances
- Supplier Score Cards
- Inspections by Measureing Device ID and Inspection Date Range
Non Conformance Process Action Management
- Identify Action, Action Owner, and Action Due Date
- Manage Open Actions
- Track Action Response Times
Comprehensive Searching Options
- Customers
- Non Conformance Reporting Date Range
- Part Numbers
- Suppliers
- Defect Types
- Work Centers / Departments
- Disposition Type - Rework / Scrap / Return To Vendor, Use As-Is
A Documented Procedure of the Non Conformance Process is 1 of 6 Mandatory Procedures for ISO 900x and AS9100.
How Manufacturing Process Non Conforming Material and Parts Matters.
That is why a Non Conformance Module is included in SimpleManufacturing™!

Ready for an Effective Solution to Your Non Conformance Process?
Reach out to us today to discuss how our Nonconforming Material Process can transform your organization’s defective material process into an Effective and Efficient task.
From free demos to customization discussions, we’re here to help you take your ISO or AS9100 nonconforming material process to the next level.