Work-In-Process / Final Inspection / Audit – NonConformances
Work In Process NonConformances
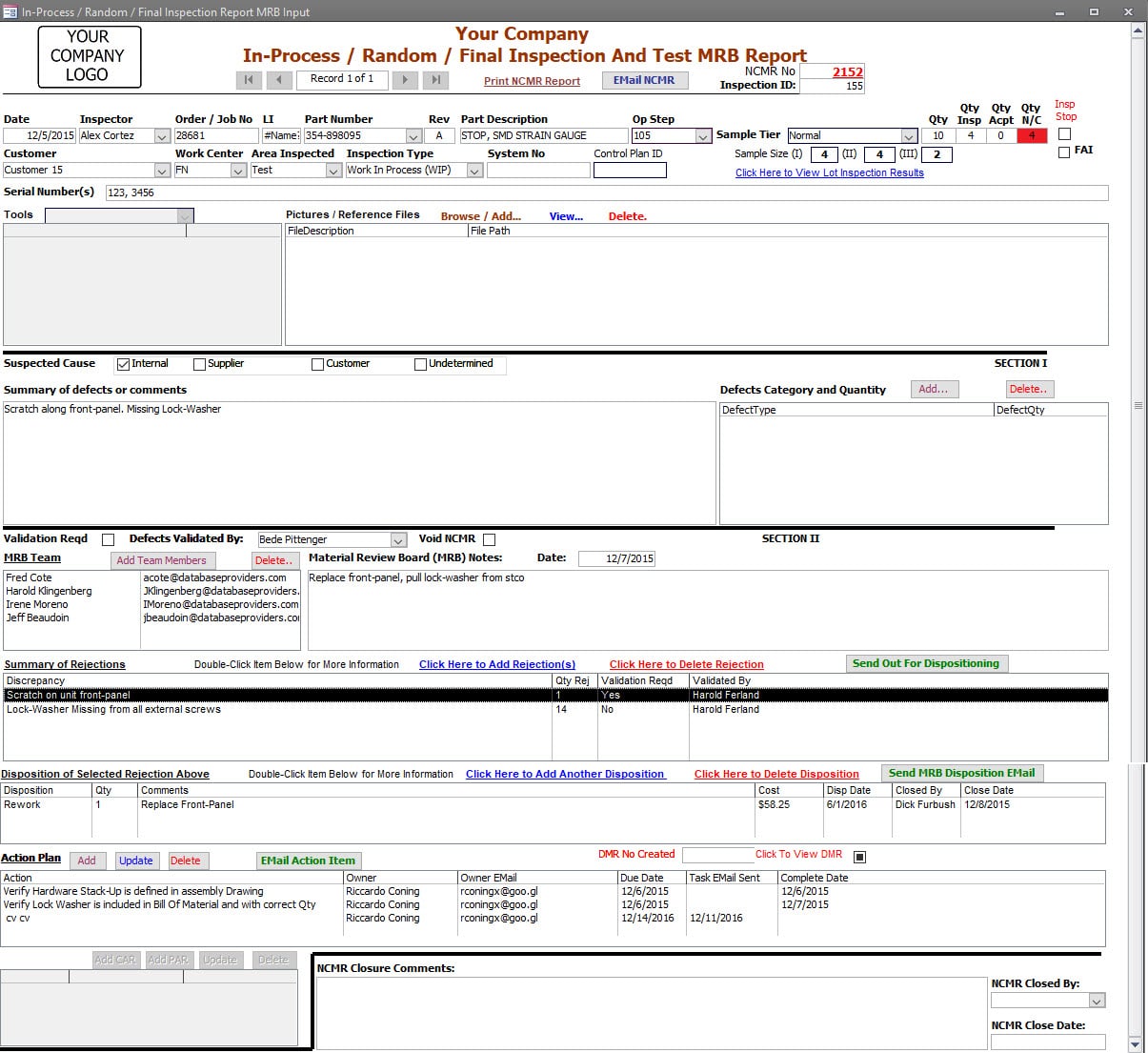
For Non-Conforming Material, the inspection record is issued and unique NCMR ID when a discrepancy is identified.
The Defect Category and Quantity can be captured for Pareto Chart reporting purposes.
Additional files can be linked to the inspection record for the non-conformance, such as red-lined drawings, and pictures of non-conformances.
A MRB Team can be identified.
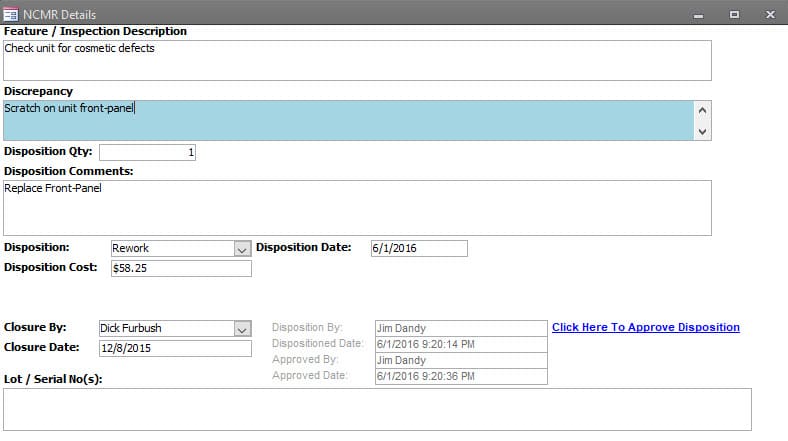
All Rejections within the inspected lot are documented within the inspection record. Each Rejection can have Multiple Dispositions and those dispositions are identified. (e.g. some quantity can be disposed to be reworked internally due to production demand, and the rest of quantity can be dispositioned to return to the vendor for repair or replacement.)
One-Click process to Generate and Send Email requesting Dispositioning, or to those Responsible for Executing the Disposition.
Actions (task items) can be entered and assigned to specific parties (individual) throughout the process. Open Action Items are visible in the Dashboard.
In the event a Corrective Action is warranted, One-Click process creates a Corrective Action and Links the corrective action record to the Inspection Record.
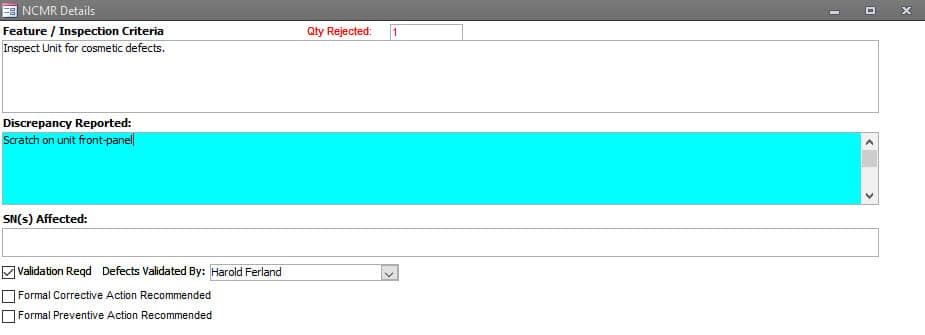
Work In Process Inspection Rejection
Rejection Information contains the Expected Condition and the Discrepancy.
When required, Serial Numbers can be added to the rejection record.
Rejections can be flagged for validation of the discrepancy with provisions to record who performed the validation.
If a Rejection warrants a corrective or preventive action, that can also be flagged for creation.